Upside foods is a fast-growing start-up working on developing cell-cultured meat for the consumer market. They have a robust R&D pipeline supported by varied teams and processes. Similar to traditional life science organizations, Upside is required to comply with regulatory requests and protocols including those from the FDA and USDA.
Upside Foods utilize the Biologics LIMS to help answer key questions including:
- How do various growth conditions affect tissue growth?
- What combination of production variables produces the best tasting and healthiest cell-cultured meat?
Answering these questions begins with gathering data in the lab from assays being continuously run by scientists. Prior to LabKey, Upside was using an assay data capture solution consisting of many connected moving parts including spreadsheets, manual processes, data transformations, databases and network drives. This solution was difficult to support and required lots of replication of data that was shaped differently depending on where it was stored. This system lacked:
- Central management and storage of their data
- Audit logging and alerts for each component and the system as a whole
- Redundancy and disaster recovery
- Scalability to meet growth needs and increasing R&D complexity
In selecting the Biologics LIMS, Upside was aiming to keep up with their many changing requirements including new instruments and assays, increasing business logic complexity and the need for a single source of truth for their data. LabKey has a number of ways to capture data including bulk import through the UI. However, Upside wanted to reduce the time and effort it took for uploading data even further, especially from high-throughput assay pipelines. They wanted to make the process as effortless as possible for their scientists. With low latency in getting instrument data loaded in the system, scientists could make faster decisions and trust the data being used for their analyses.
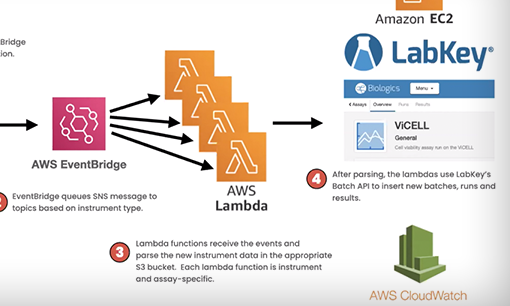
Presented by Jay Kasberger, Senior Manager, Software Engineering at Upside Foods, the video below describes how Upside Foods built a pipeline that leverages AWS to automate the flow of assay data. By using Lambda and DataSync, Upside Foods reduced the work scientists have to perform to transfer runs and results into the system while keeping thorough logs and automatically alerting to any issues.
Click here to learn more about the Biologics LIMS.
Watch the presentation below:
[vc_video link=”https://www.youtube.com/watch?v=N3sIgSjshx8″]

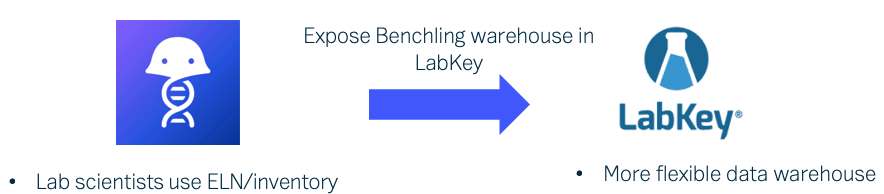



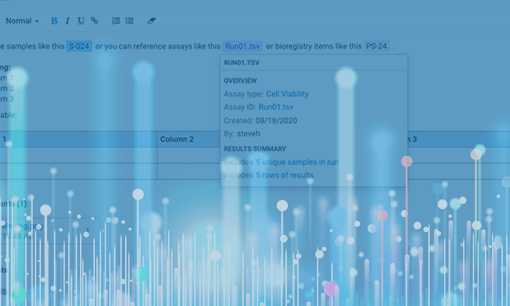
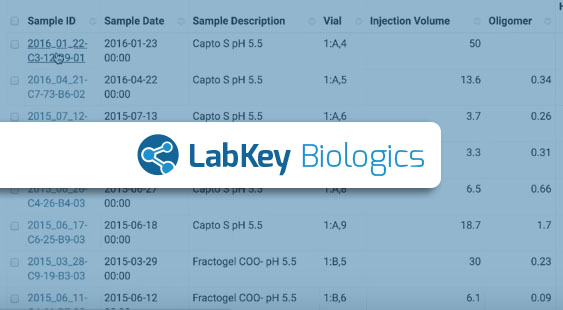

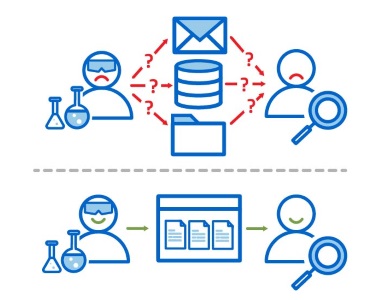 Using a data sharing hub, team members never need to wonder where to find the data they need. Clear and consistent gathering of experiment metadata means that not only do they see what they need, they immediately know when it’s ready for the next task or experiment. With LabKey Biologics, team members share access to the entity designs, biological samples, and analytical results they need for further research.
Using a data sharing hub, team members never need to wonder where to find the data they need. Clear and consistent gathering of experiment metadata means that not only do they see what they need, they immediately know when it’s ready for the next task or experiment. With LabKey Biologics, team members share access to the entity designs, biological samples, and analytical results they need for further research.