Project Background
In 2017, Harvard Pilgrim Health Care Institute (HPHCI) was selected by the U.S. Food and Drug Administration (FDA) through the FDA-Catalyst program to lead the development of a mobile application, called FDA MyStudies, that would facilitate the collection of real-world data directly from patients to support clinical trials, observational studies, and registries. The effort was funded by an award to FDA scientific staff from the Patient Centered Outcomes Research Trust Fund which is administered by the Associate Secretary for Planning and Evaluation (ASPE) of the Department of Health and Human Services. Harvard Pilgrim selected the mobile application development firm Boston Technology Corporation (BTC) and LabKey as their development partners for the project. BTC was tasked with developing a user friendly mobile interface while LabKey was tasked with building a secure back-end storage environment for collected data.
Why LabKey
LabKey Server was selected as the back-end data management solution for this project for a number of key reasons, one of which being the platform’s flexible, science-specific architecture. With the project’s long-term goal of expanding the use of real-world data across research programs, the application framework needed to support a broad range of potential healthcare topics through configuration as opposed to requiring development for each new project.
LabKey Server also stood out as an ideal solution because of the platform’s ability to handle PHI/PII data in a manner compliant with HIPAA and FISMA regulations. Finally, one of the project requirements outlined by the FDA was that the resulting application and storage architecture would need to be made available as open source to the scientific community. LabKey Server, an open source platform licensed under Apache 2.0, was able to support this distribution model without any changes to the existing licensing model.
The Implementation
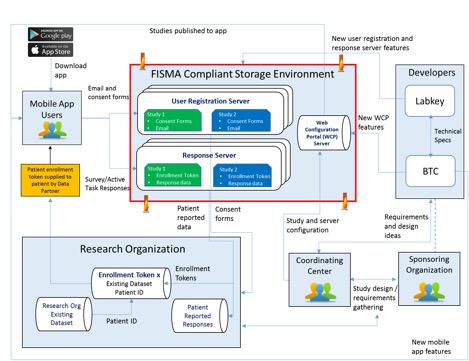
The back-end storage environment is composed of three independent web applications:
- Response Server: used to store data captured via the mobile application and provide secure access to these data for data analysis purposes.
- Registration Server: used to manage participant authentication, preferences, notifications, and consents.
- Web Configuration Portal: used to design study questionnaires and store study configuration information including consent forms, eligibility tests, surveys, and study resources.
This dispersed data model ensures secure partitioning of all identifying information from response data, helping ensure patient privacy. LabKey Server provides role-based governance of the data stored on the Registration and Response servers and ensures that data are only accessible by authorized users. When it comes time for analysis, data stored in the response server can be accessed by authorized users via a number of different methods including LabKey’s built in analytics capabilities, download to SAS or R, or export to Excel or other standard format.
The bulk of the components used in the development of the secure data storage environment were previously existing in the LabKey Server platform. However, three key areas of custom development and extension were required to support the project’s use:
- Enrollment Tokens: A unique token that is assigned to each participant upon registration that can be used to restrict their enrollment to a specific study cohort, as well as match the collected study data to external systems (e.g., EHRs).
- Automatic Schema Creation: Automatic generation of a new database schema when a study questionnaire is created, eliminating the need for manual schema development.
- Mobile App Response Handling: Capabilities to support automated parsing of the JSON responses sent by the mobile application were implemented, enabling the storage of results in the schema, in a scalable manner.
The LabKey team delivered these developments in a custom module using an agile development methodology, refining them based on client feedback in tandem with the development of the mobile application UI.
Results
To evaluate the usability and viability of the application and data storage environments, Harvard Pilgrim contracted with Kaiser Permanente Washington Health Research Network (KPWHRN) to launch a pilot study examining the medication use and healthcare outcomes of pregnant women throughout their pregnancy. For the pilot program, the Harvard Pilgrim team utilized LabKey’s Compliant Cloud hosting services to manage the storage of study data in a secure AWS cloud environment. Participants who successfully completed the study reported high levels of usability and comfort sharing sensitive information using the app. The pilot was deemed a success, and in Fall 2018 the FDA released the open source code and documentation publicly for use in other studies. Since its release, the FDA MyStudies platform has been selected to support a clinical trial as well as a disease registry.
Webinar Presentation
On May 9, 2019, subject matter experts from the FDA, HPHCI, BTC, and LabKey, presented an overview and many details about this project in a live webinar entitled: An Introduction to the FDA MyStudies App: An Open-Source, Digital Platform to Gather Real World Data for Clinical Trials and Research Studies.
Related Reading
https://www.fda.gov/downloads/Drugs/ScienceResearch/UCM625206.pdf
