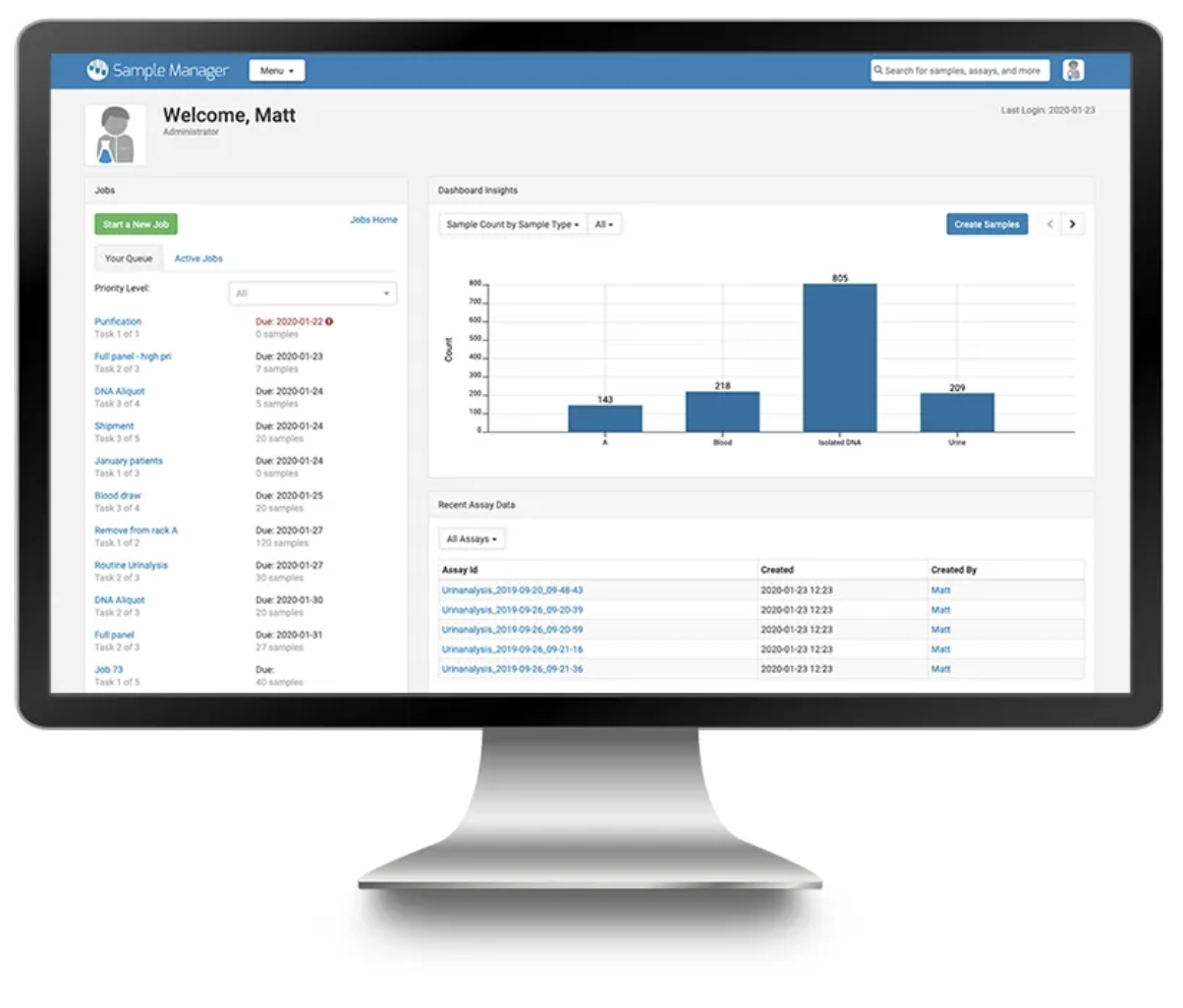
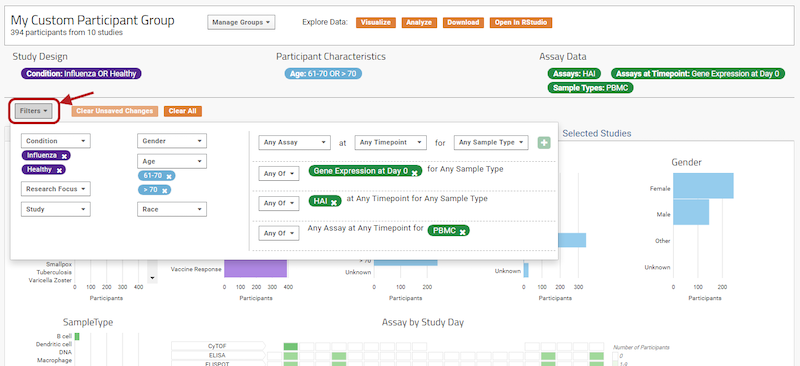
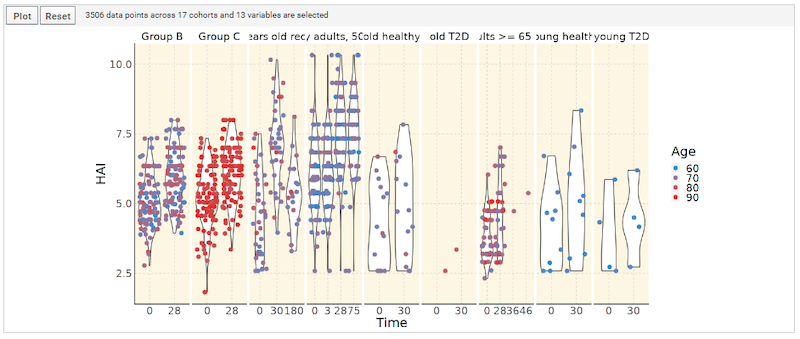
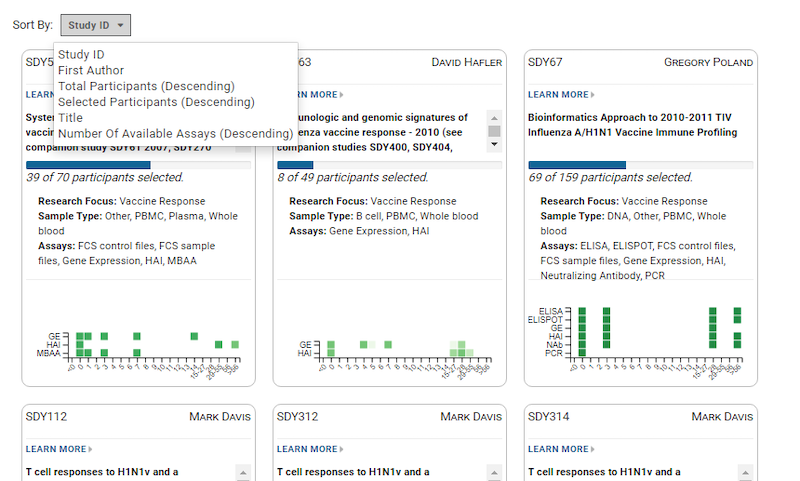
The 2020 LabKey User Conference featured informative sessions showing how LabKey is being used by a variety of life science research organizations, as well as educational talks from the LabKey Team. Conference highlights include:
- Watch presentations from NIAID/NIH, Nestlé Research, Just-Evotec Biologics, and Knight Cancer Institute showing how they have partnered with LabKey
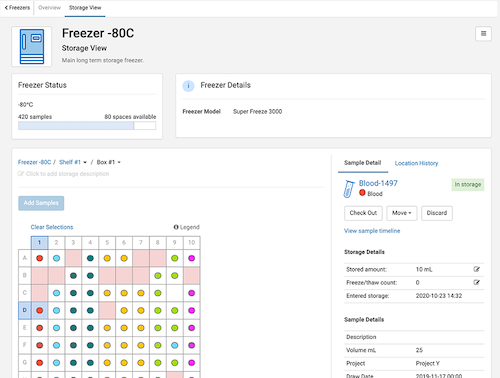
- See previews of our upcoming Biologics Electronic Lab Notebook (Day 2), and our Freezer Management solution within LabKey Sample Manager (Day 3)
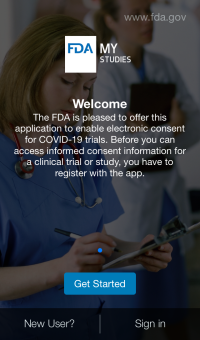
- Hear about LabKey MyStudies (Day 4)- an open-source system for conducting IRB-approved clinical trials, collecting patient reported outcomes, and obtaining informed consent using mobile devices
- Learn from our developers with our Tech Talks (Day 4) to get a deep dive into more technical areas of the platform
[vc_custom_heading text=”Have questions? Want more information?” font_container=”tag:h2|text_align:center” use_theme_fonts=”yes”][vc_btn title=”Contact Us” color=”green” align=”center” link=”url:https%3A%2F%2Fwww.labkey.com%2Fabout%2Fcontact%2F”][vc_separator][vc_row equal_height=”yes”][vc_custom_heading text=”Day 1, October 6th” use_theme_fonts=”yes”][vc_video link=”https://youtu.be/HiT_CLKG7Bs”][vc_btn title=”Watch on YouTube” color=”green” align=”left” link=”url:https%3A%2F%2Fyoutu.be%2FHiT_CLKG7Bs”]Introduction
Presented by Michael Gersch, CEO, LabKey
00:11:55 – LabKey Server Product Update
Presented by Adam Rauch, VP of Product Strategy, LabKey
00:28:27 – HGRepo Informatics Framework for the NIAID/DIR COVID-19 Immune Response Consortium
Presented by Sandhya Xirasagar Ph.D., Lead, Clinical and Laboratory Informatics Section, NIAID & Jason Barnett, Contract Lead, Medical Science and Computing, Inc.
00:54:35 – Assay and Study Management with LabKey Server
Presented by Bernhard Sonderegger Ph.D., Senior Specialist Data Systems and Management, Nestlé Research
01:15:00 – Reporting and Visualizations via ODBC Integrations
Robert Morphew, Technical Program Manager, LabKey[vc_separator][vc_row equal_height=”yes”][vc_custom_heading text=”Day 2, October 7th” use_theme_fonts=”yes”][vc_video link=”https://youtu.be/_0j5TTzB1NI”][vc_btn title=”Watch on YouTube” color=”green” align=”left” link=”url:https%3A%2F%2Fyoutu.be%2F_0j5TTzB1NI”]LabKey Biologics Update
Presented by Bernie Lee, Product Manager, LabKey
00:28:52 – ELN (Electronic Lab Notebook) Preview
Presented by Bernie Lee, Product Manager, LabKey
00:56:54 – The Quest for an ELN We Actually Want To Use
Presented by Tara Kulas, Scientist, Analytical Sciences, Just – Evotec Biologics
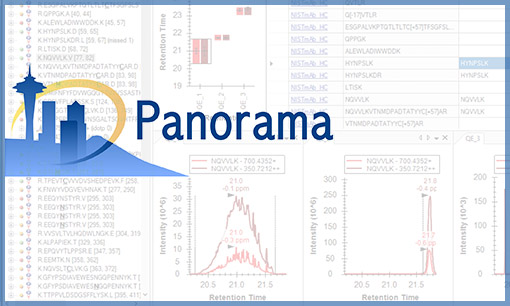
01:26:38 – Introduction to Panorama and LabKey Support for Targeted Mass Spectrometry
Presented by Josh Eckels, VP of Engineering, LabKey[vc_separator][vc_row equal_height=”yes”][vc_custom_heading text=”Day 3, October 8th” use_theme_fonts=”yes”][vc_video link=”https://youtu.be/bolH_k0YQq0″][vc_btn title=”Watch on YouTube” color=”green” align=”left” link=”url:https%3A%2F%2Fyoutu.be%2FbolH_k0YQq0″]LabKey Sample Manager Update
Presented by Hannah Brakke, Product Manager, LabKey
00:26:09 – Freezer Management Preview
Presented by Hannah Brakke, Product Manager, LabKey
00:57:02 – Data Management in Precision Oncology
Presented by Patrick Leyshock, Ph.D., Knight Cancer Institute, Oregon Health & Science University
01:27:20 – Using LabKey Server with Sample Manager
Presented by Steve Hanson, Director of User Education, LabKey[vc_separator][vc_row equal_height=”yes”][vc_custom_heading text=”Day 4, October 9th” use_theme_fonts=”yes”][vc_video link=”https://youtu.be/ikEeVwjPa24″][vc_btn title=”Watch on YouTube” color=”green” align=”left” link=”url:https%3A%2F%2Fyoutu.be%2FikEeVwjPa24″]LabKey MyStudies: Supporting Mobile E-Consent, Study Design and Infrastructure for Clinical Trials
Presented by Adam Rauch, VP of Product Strategy, LabKey
00:30:26 – Automate Data Import and Processing using File Watchers
Karl Lum, VP of Engineering, LabKey
01:00:26 – Using LabKey Artifacts for your Build Process
Presented by Susan Hert, LabKey
01:28:53 – JavaScript Development within LabKey Server
Presented by Nick Kerr, LabKey
01:59:39 – Bringing Your Data Together
Presented by Binal Patel, LabKey
02:30:19 – LabKey Cloud Hosting and Best Practices
Presented by Stuart MacDonald and Isa Farnik, LabKey[vc_separator]